Environmental Design Engineering
To establish the sound material-cycle society, our laboratory is aiming at developing procedures to analyze, manage, design and control treatment and disposal systems for solid wastes, including recycling and resource recovery, by applying techniques based on disciplines of Transport Phenomena, Environmental Systems Engineering and Environmental Chemical Engineering. By performing fundamental and applied experiments in both laboratory and field scales and using computer analysis and simulation, We approach the following subjects;
- Analysis on material and energy flows in urban metabolism systems,
- Planning, designing, analysis and control of environmental treatment plants, and
- Evaluation and optimization of environmental systems centering human life space.
Academic Staff
Masaki TAKAOKA
 Professor (Graduate School of Engineering)
Professor (Graduate School of Engineering)
Research Topics
- Solid waste management
- Characterization of solid waste
- Energy and resource recovery through thermochemical conversion of waste
- Development of carbon neutral emission-oriented waste treatment systems
- Recovery of valuable metals from waste
- Hazardous waste treatment and management
- Treatment of waste contaminated with radioactive substances
Contacts
Katsura Campus, C-cluster, C1-3, Room 461
TEL: +81-75-383-3335
FAX: +81-75-383-3338
E-mail: takaoka![]() epsehost.env.kyoto-u.ac.jp
epsehost.env.kyoto-u.ac.jp
Kazuyuki OSHITA
 Associate Professor (Graduate School of Engineering)
Associate Professor (Graduate School of Engineering)
Research Topics
- Development and characterization of advanced sewage treatment system
- Dewatering and drying of sludge, and remediation of contaminated sediment using liquefied dimethyl ether at normal temperature
- Study on proceeding of the sewage sludge utilization for energy
- Separation and recovery of valuable matter from solid waste
Contacts
Katsura campus, C-cluster, C1-3, Room 463
TEL: +81-75-383-3336
FAX: +81-75-383-3338
E-mail: oshita![]() epsehost.env.kyoto-u.ac.jp
epsehost.env.kyoto-u.ac.jp
Research Topics
Characterization of solid waste
To develop new technologies, the detailed characterization of target substances is essential. If chemical state of heavy metal in solid waste becomes clear, we can select the most reasonable treatment or recycling technologies for this solid waste. Because the toxicities of heavy metal are different by the chemical states of the heavy metal, understanding of chemical states of heavy metal in solid waste is very important to evaluate the toxicity and the release of heavy metal to environment.
We proceed now the direct speciation of heavy metal in municipal solid waste incinerator fly ash using X-ray Absorption Fine Structure(XAFS) in the SPring-8, which is the world's largest third-generation synchrotron radiation facility, provides the most powerful synchrotron radiation currently available.
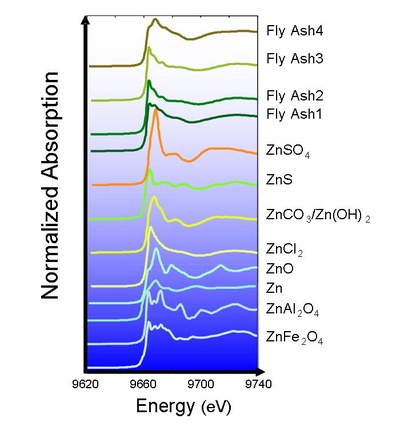
Figure 1 XAFS spectrum of zinc compounds in MSWI fly ashes.
Construction of waste/resource recycling system for the realization of a decarbonized society and regional circular and ecological sphere
In the field of waste/resource recycling, if we do not consider a drastic approach such as innovation of individual treatment technology and reconstruction of recycling/treatment system toward the realization of a decarbonized society and regional circular and ecological sphere, it is difficult to achieve virtually zero GHG emissions by the middle of the 21st century. In addition, waste is a mass of energy and resources, and waste recycling/treatment facilities can be an energy and resource supply base in each region. Since the technologies and systems have different constraints such as natural conditions and economic conditions, it is necessary to conduct various studies according to each country/region and pave the future way. In this research, we aim to develop waste/resource recycling technology for the realization of a decarbonized society and regional circular and ecological sphere, and to propose a sustainable society that connects arteries and veins of urban infrastructures.
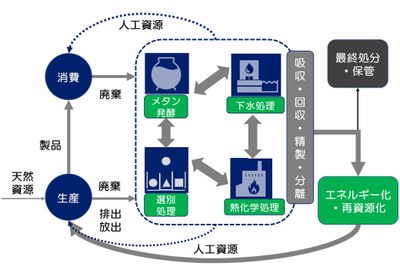
Figure 2. Waste/resource recycling system flow
Dewatering and drying of sewage sludge, and remediation of PCB-contaminated sediment using liquefied dimethyl ether at normal temperature
Sewage sludge is generated by treating wastewater in wastewater treatment facilities and categorized as industrial waste in Japan. And sediments are muddy matter at the bottom of river, lake and harbor, and sometimes severely contaminated with dioxins and polychlorinated biphenyls (PCBs). Because both of them are generated in large amounts with human activity, they are necessary to reduce their volume and to remediate certainly and efficiently.
We have been suggested and developed the new dewatering, drying and remediation process of sewage sludge and contaminated sediments using liquefied dimethyl ether (DME) as water and organic pollutants extraction agent. DME is the simplest available ether and is a gas at normal temperature and pressure. Its standard boiling point is –25℃, and it liquefies at six times atmospheric pressure at normal temperatures. Moreover, some water and organic pollutants: dioxins and PCBs, dissolves in DME.
There are three major advantages of this method:
- Since it does not use any heat, the energy requirement is almost half that of classical techniques. In addition, no processing of exhaust gases is required.
- After dewatering, liquefied DME can be easily evaporated by reducing the pressure, and extracted water, organic pollutant, and dewatered substance can be easily separated.
- Gaseous DME can be re-liquefied by compression and reused for dewatering. Therefore, it can be used repeatedly.
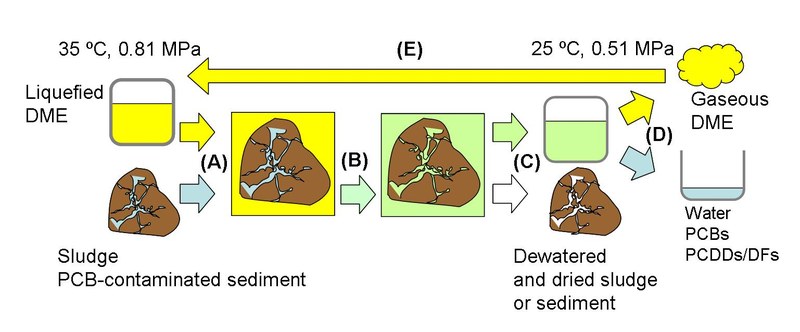
Figure 3 Concept of dewatering and remediation process of sludge or contaminated sediment using dimethyl ether (DME).
(A) Mixture of liquefied DME and sludge or contaminated sediment.
(B) Extraction of water (Dewatering and drying).
(C) Separation of water from sludge or contaminated sediment.
(D) Selective evaporation of DME by reducing pressure.
(E) Reuse by pressurization and liquefaction of DME.
